

Development of analytical methods for environmental objects, pharmaceutical, biological, food samples, and functional materials using spectroscopy, chromatography, and modern sample preparation techniques.
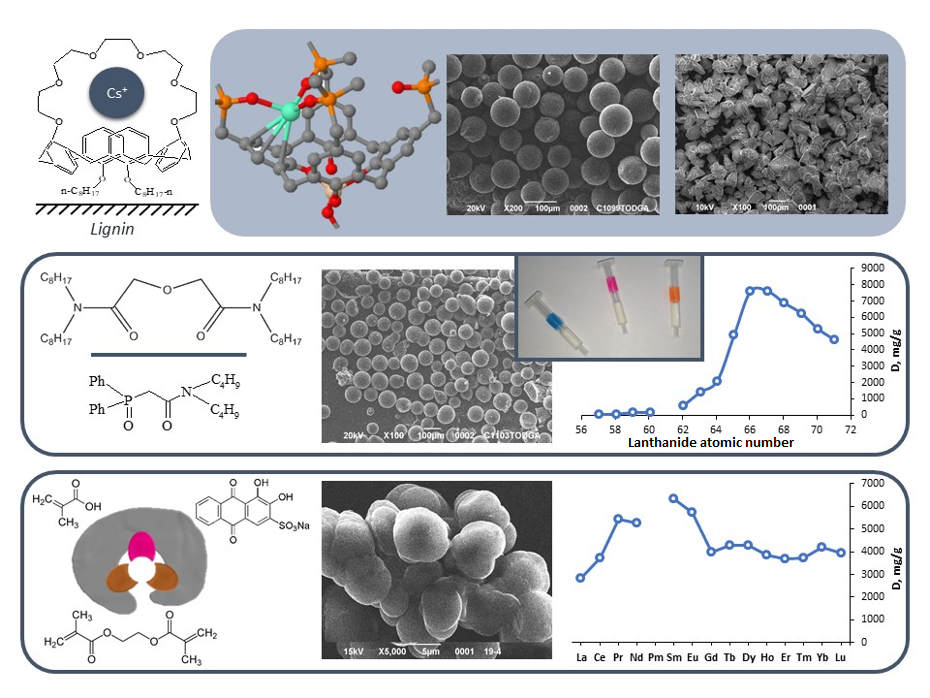
Highly selective sorption materials. Selective sorption materials are of high importance because they can remove and concentrate target substances from complex mixtures, enabling purification and separation processes. Innovative sorption materials and extraction systems with high selectivity have been developed for the analytical separation and concentration of heavy metals and radionuclides. Sorbents based on calixarene derivatives and ion-imprinted polymers ensure the efficient removal of cesium ions and rare earth elements. Calixarenes bearing various substituents on their upper and lower rims were investigated, and the influence of these substitutions on their metal-ion binding ability was elucidated. Ion-imprinted divinylbenzene/ ethylene glycole dimetacrylate and methacrylic acid copolymers with different crosslink ratios have been synthesized using trapping or chemical immobilization and their sorption properties were investigated. Extraction columns have been designed for the separation and concentration of uranium and americium, utilizing polymeric materials modified with tetraoctyldiamide of diglycolic acid and carbamoylphosphine oxides.
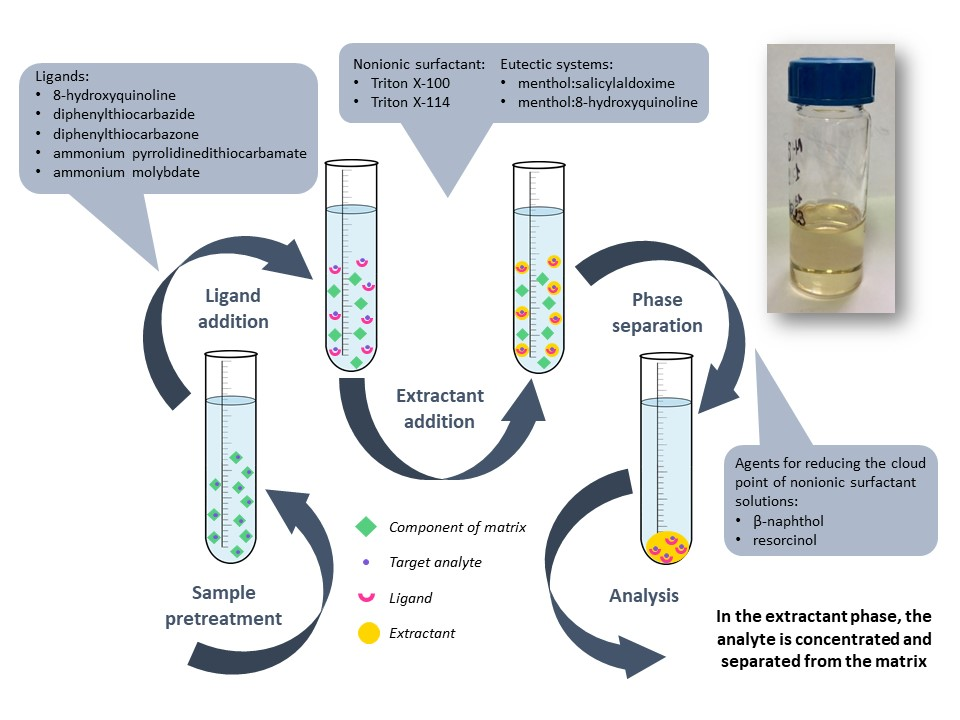
Microextraction techniques: microextraction using deep eutectic solvents and cloud point microextraction. Deep eutectic solvents (and in a broader sense, eutectic solvents) are currently being applied in various fields, including analytical chemistry, where they are proving to be effective extractants for organic and inorganic compounds. They are mixtures of substances that form an eutectic with a melting point lower than that of the individual components. The main principle of formation of such systems is the ability of their components to intermolecular interaction, such as hydrogen bonding, whereby they are considered as supramolecular complexes in the liquid state. The academic and technological interest to (deep) eutectic solvents is associated with a number of advantages, such as the possibility of utilizing safe and biodegradable substances, easiness of preparation, economical profitability, and the potential for governing functional properties. This scientific direction of the Department is aimed on the creation and application of extraction systems based on hydrophobic (deep) eutectic solvents to improve the effectiveness of sample preparation prior to spectral analysis. A range of new menthol-based (deep) eutectic solvents was obtained, exhibiting diverse solid–liquid phase behavior. The study of their extraction properties confirmed their suitability as extraction media for removing various metal ions. Furthermore, the results contribute to the development of strategies for discovering new designer solvents.
Cloud point microextraction is another technique widely used in modern analytical chemistry. It is based on the ability of nonionic surfactant solutions to separate into aqueous and micellar phases under particular conditions. A new separation and preconcentration procedures based on cloud point extraction were developed for arsenic, cadmium, lead and mercury trace analysis using inductively coupled plasma optical emission spectrometry. The combination of Triton X-100 with salting-out agents and complex forming ligands was investigated as an extraction system for trace elements quantification. New agents for reducing the cloud point of nonionic surfactant solutions were proposed. The procedures suggested allow eliminating spectral interferences induced by matrix components of pharmaceuticals as well as lowering detection limits of the method. They can be effectively applied for analysis of pharmaceutical products according to ICH Q3D Guideline.
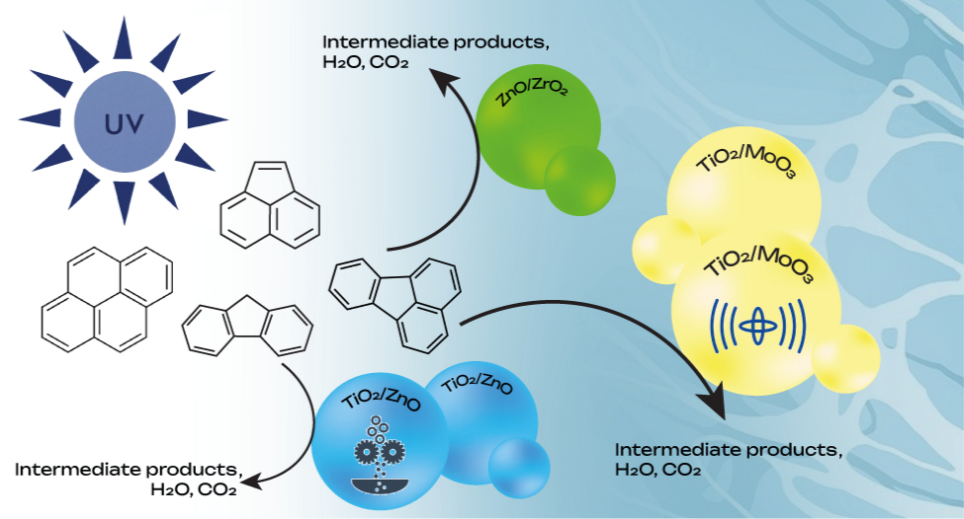
Photocatalytic remediation of xenobiotics. Polycyclic aromatic hydrocarbons (PAHs) rank among the most dangerous environmental pollutants due to their high toxicity, carcinogenic nature, and tendency to accumulate in ecosystems. Developing effective methods for their neutralization remains a highly urgent challenge in modern science. Efficient photocatalytic materials based on metal oxides (TiO2, ZnO with heterojunctions) capable of degrading PAHs in aqueous solutions under ultraviolet irradiation have been developed. The simultaneous degradation of PAH mixtures under the action of the developed photocatalysts was investigated using gas chromatography-mass spectrometry (GC-MS). Studies of the degradation kinetics and identification of intermediate reaction products allow us to optimize the composition and processing methods of the catalysts to achieve maximum efficiency in water purification processes from hazardous organic pollutants.
Grants
Projects funded by the National Academy of Sciences of Ukraine:
Projects supported by the National Research Foundation of Ukraine: