Synthesis and investigation of heterocyclic compounds, natural molecules, and supramolecular systems for applications in medicinal chemistry, agro-industry, and other fields.
One of the main scientific research directions of the Department is Diversity Oriented Synthesis using different approaches and strategies:
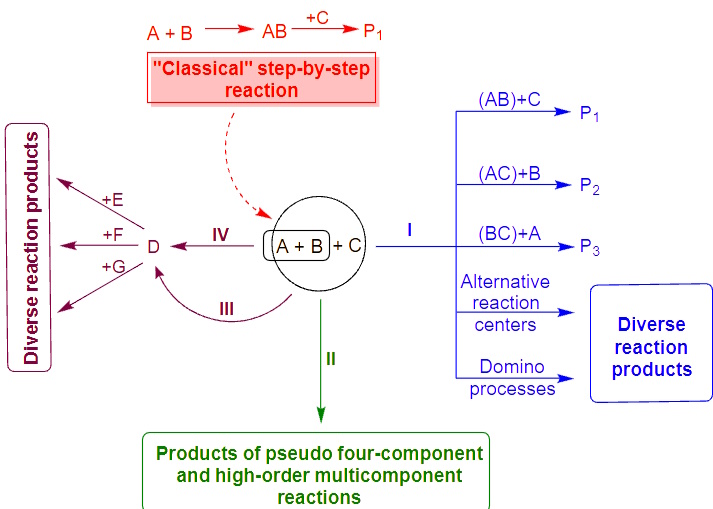
For instance, using thermal heating and non-classical activation methods – microwave and ultrasonic irradiation – a strategy of chemocontrolled multicomponent condensations was developed and applied to the synthesis of various types of heterocyclic compounds (Conditions-based Divergence Strategy). On this basis, multicomponent reactions of polynucleophiles and carbonyl compounds were proposed, the direction of which is controlled by varying the reaction parameters, enabling the selective preparation of up to five different types of heterocyclic systems from the same chemotypes of reagents.
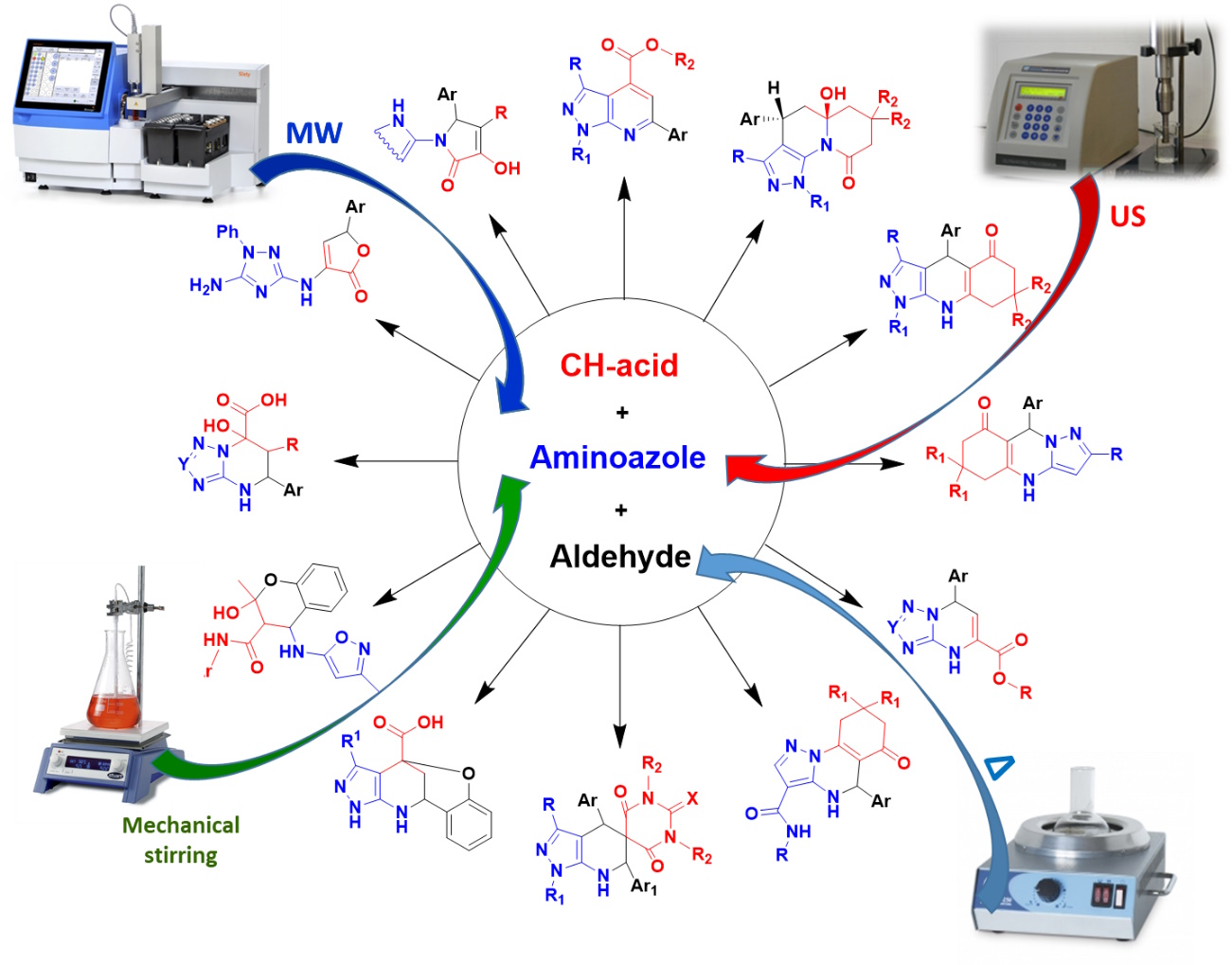
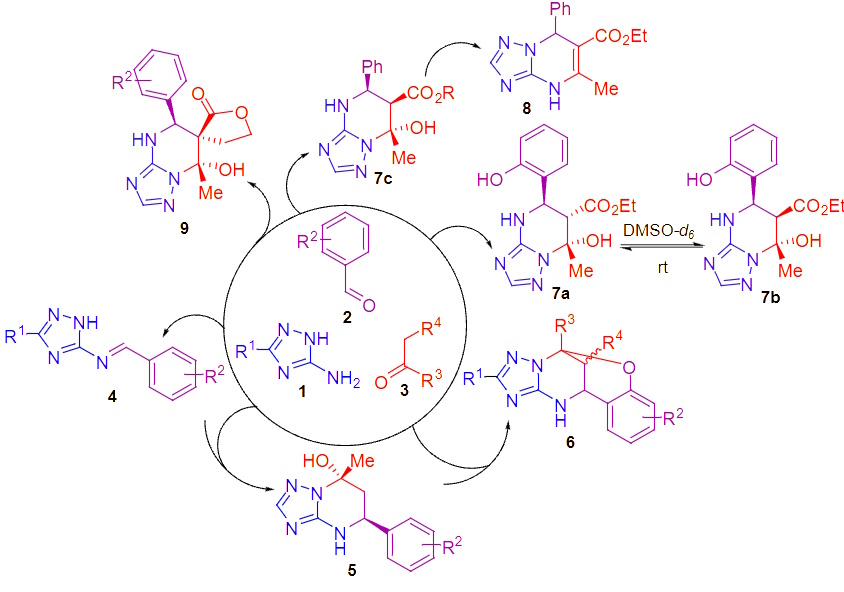
Search for artifacts in the fields of chemical transformations, inspired by the Bignelli multicomponent condensation using 3-amino-1,2,4-triazole as a heterocyclic equivalent or urea. We develop conditions that enable high regio- and stereoselectivity in the formation of alternative products using green solvents such as water or ethanol. In microwave synthesis, certain transformations are carried out in closed vessels at temperatures far above the boiling point of the solvents. The structure of the final molecules, including the stereochemical aspects, is determined using an arsenal of 2D NMR spectroscopy and X-ray analysis.
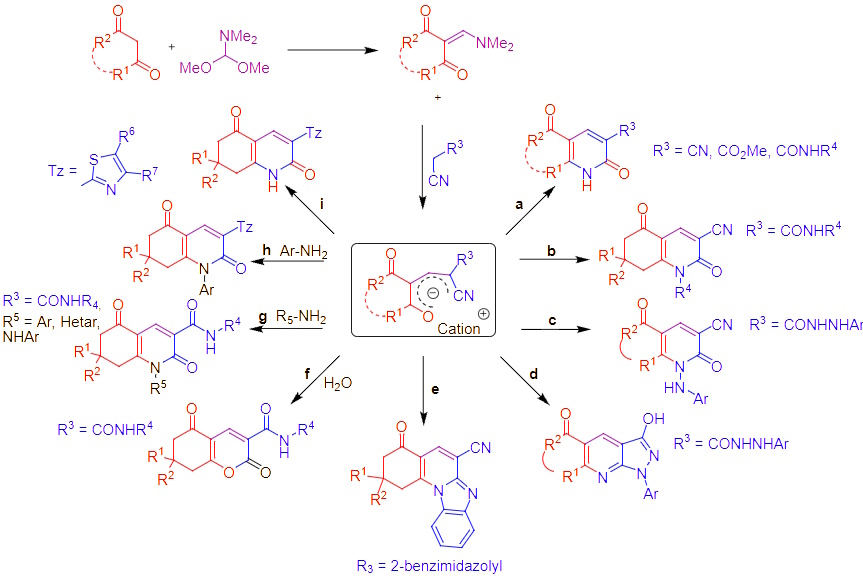
The 2-(2-cyanovinyl)-3-oxo-cyclohex-1-enes are versatile reactive intermediates that can be "trapped" and used in multidirectional transformations to synthesize a variety of complex molecules. This modular transformation concept allows the combination of various reagents under different reaction conditions in a single reactor, significantly expanding the possibilities of controlled multicomponent condensations.
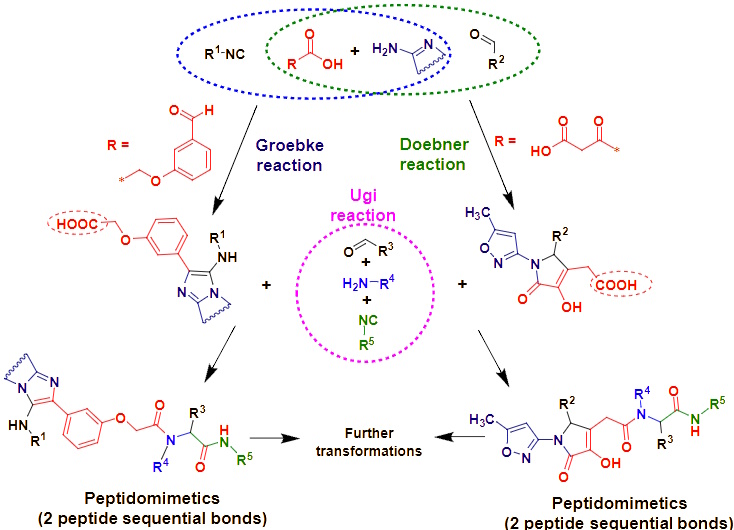
Another important field of research is the isocyanide-based multicomponent reactions for the synthesis of new biologically active hybrid molecules containing a peptidomimetic chain and an additional pharmacophoric heterocyclic moiety. The combination of several pharmacophoric fragments in one compound can potentially increase biological activity. For instance, the tandem combination of Doebner or Groebke type multicomponent reactions with an Ugi reaction allows the introduction of a heterocyclic fragment into the structure of peptidomimetics.
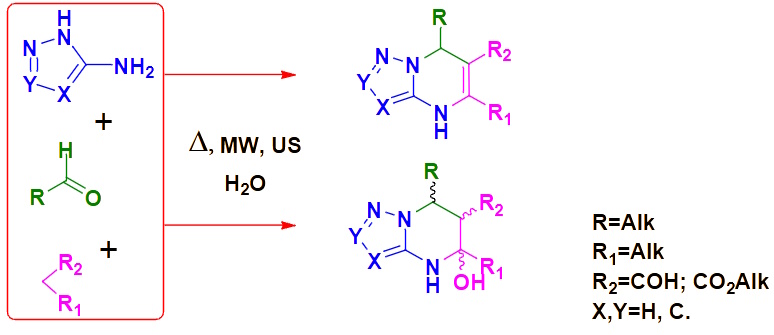
The synthesis of partially hydrogenated azolopyrimidinescontaining a nodal nitrogen atom and bearing aliphatic substituents, is a source of new building blocks for the targeted design of potential drugs. The multicomponent synthesis of such building blocks makes it possible to obtain previously unavailable compounds with low molecular weight and reaction centers for further chemical modifications. This approach is based on the principles of "green chemistry" (use of non-toxic solvents) and non-classical methods of activation.
In addition, an important direction is the synthesis and study of molecular structure and conformational changes by various physicochemical and spectral methods of spiro-organized nitrogen-containing heterocycles to solve problems in medicinal and pharmaceutical chemistry.
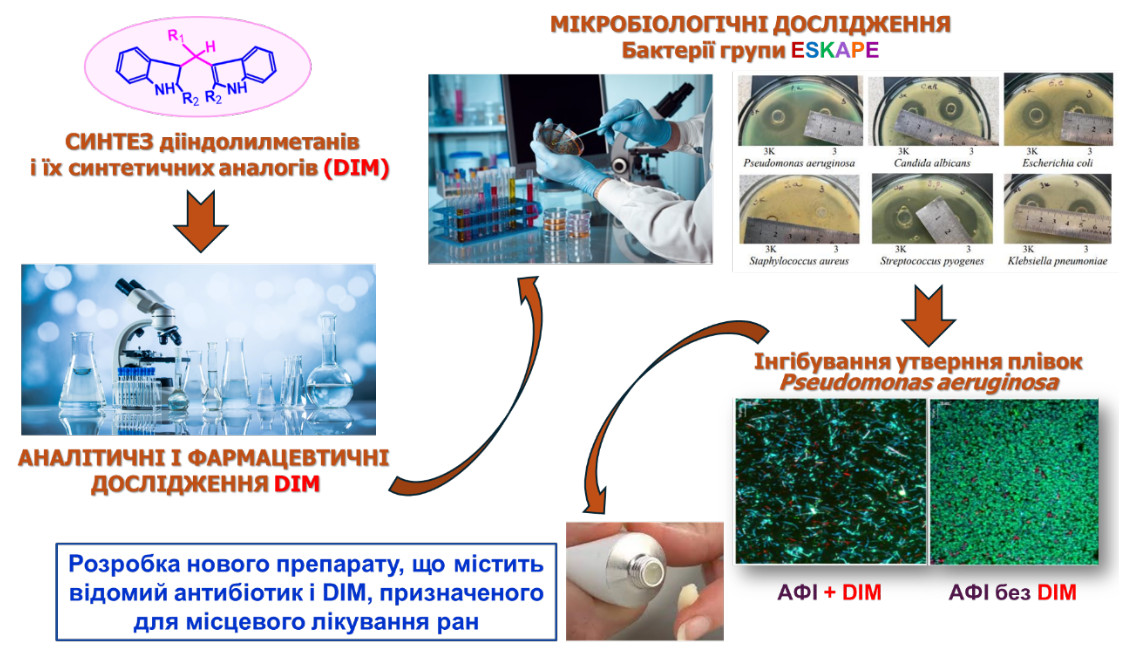
The natural compound diindolylmethane (DIM) and its synthetic analogues are of great interest due to their broad spectrum of pharmacological activity. In particular, some of these compounds have shown the ability to enhance the antibacterial efficacy of known antibiotics against strains of microorganisms that are resistant to these antibiotics. This discovery forms the basis for further pharmaceutical developments of innovative drugs that combine an antimicrobial active pharmaceutical ingredient with DIM to provide effective antimicrobial activity against multidrug-resistant microorganisms.
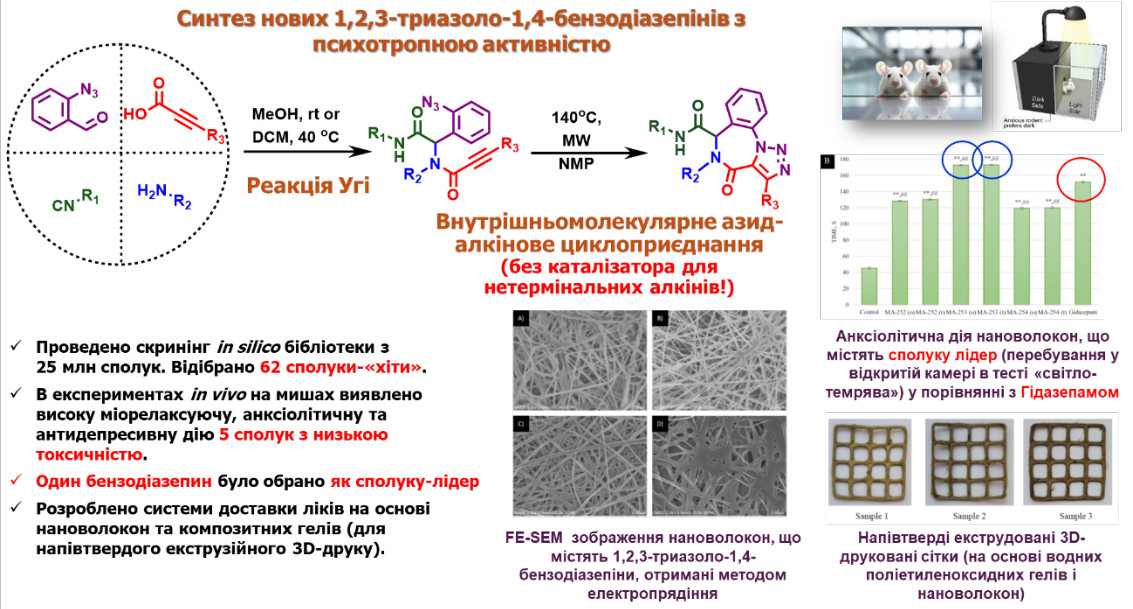
A microwave-assisted method of synthesis of a brand-new 1,2,3-triazolo-1,4-benzodiazepines exhibiting significant psychotropic activity was developed. Following comprehensive molecular docking studies across an extensive library of these heterocycles, five ‘hit’ compounds were selected and synthesized. The psychotropic properties of these compounds were evaluated in vivo using a variety of behavioural assays in mice. Results demonstrated that the selected compounds possessed muscle relaxant and anxiolytic effects comparable to the reference drug Gidazepam, without impairing motor coordination and with enhanced endurance. Additionally, their antidepressant efficacy exceeded that of Imipramine. To optimize bioavailability, two delivery systems were developed: polymer nanofibers obtained via electrospinning and water-polymer gels suitable for semi-solid extrusion 3D printing. Ultimately, a lead compound demonstrating optimal activity, high stability, and low toxicity was identified, along with the most suitable nanofiber-based delivery system.
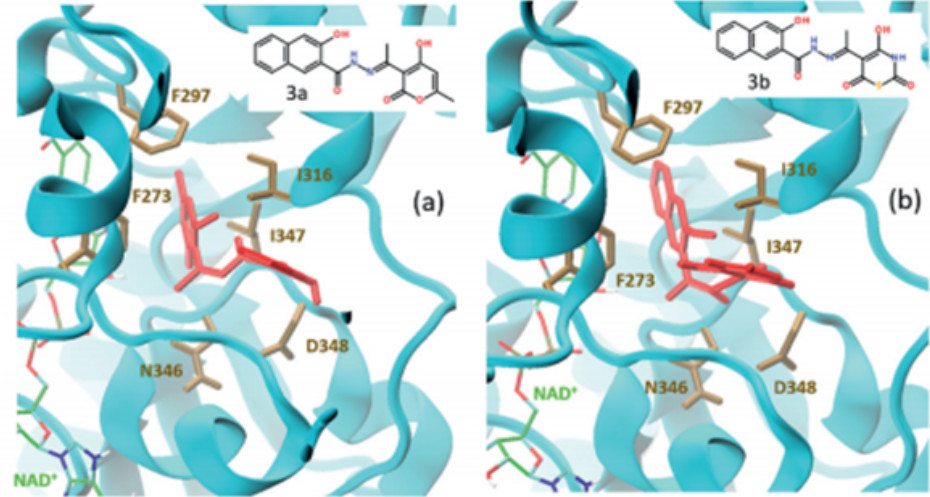
The development of new SIRT1 modulators (NAD+-dependent deacetylase enzyme) in collaboration with the company Enamine (Kyiv) is a promising direction in the fight against cancer and age-related neurodegenerative diseases. In particular, within the framework of this direction, molecular docking of new N-acylhydrazone derivatives and their analogs to the active center of the SIRT1 enzyme was performed, which allowed finding a number of "hit" compounds.
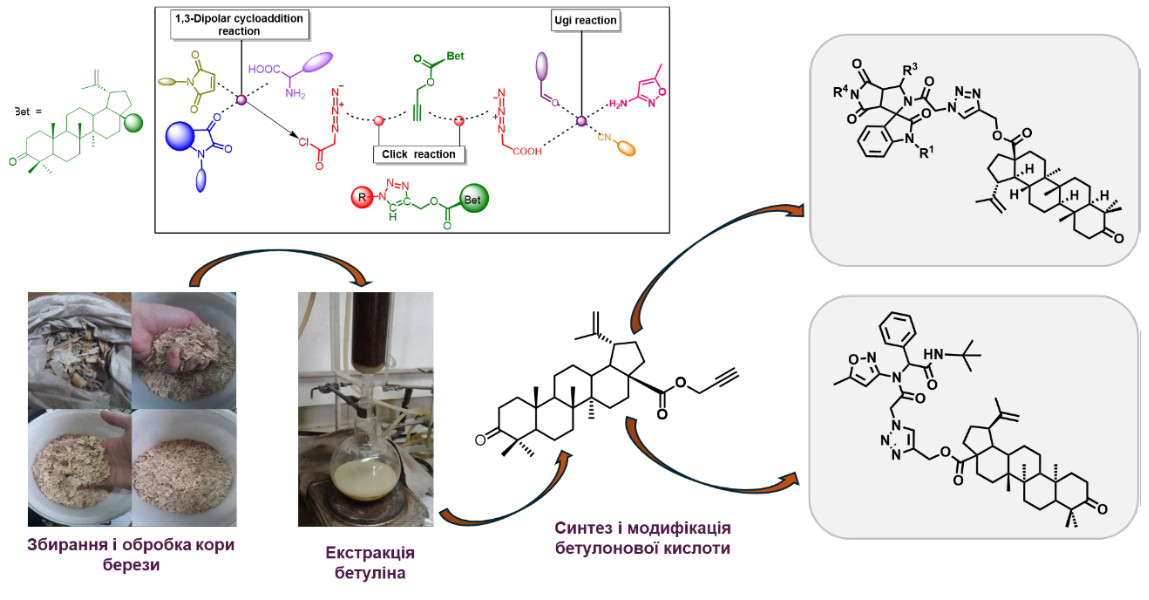
Another important direction is the modification of natural molecular platforms, in particular triterpenoids of the lupane series and steroids to solve problems in materials science and medicinal chemistry. Such hybrid compounds have been synthesized in particular for the production of "smart" gels, as well as for the search for new drugs.
Supramolecular chemistry refers to chemical systems consisting of individual molecules held together by weak non-covalent bonds. It is used in analytical chemistry, molecular recognition, catalysis, pharmaceutical research, drug delivery, and the development of new materials.
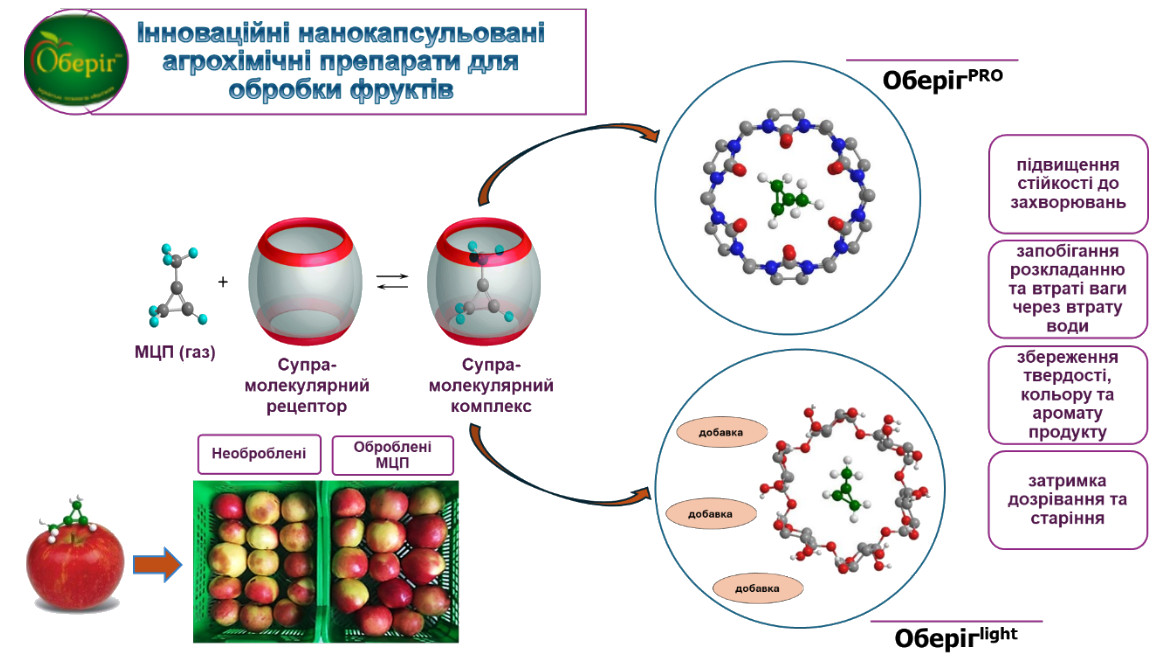
Our research in supramolecular chemistry is based on the ability to bind and preserve certain molecules in a supramolecular complex with their subsequent release under desired conditions. In particular, we have developed new agrochemicals based on supramolecular complexes of 1-methylcyclopropene for the treatment of fruits to extend their shelf life. As of today, two preparations “OberihPRO” and “OberihLIGHT ” have been introduced to the Ukrainian agromarket.
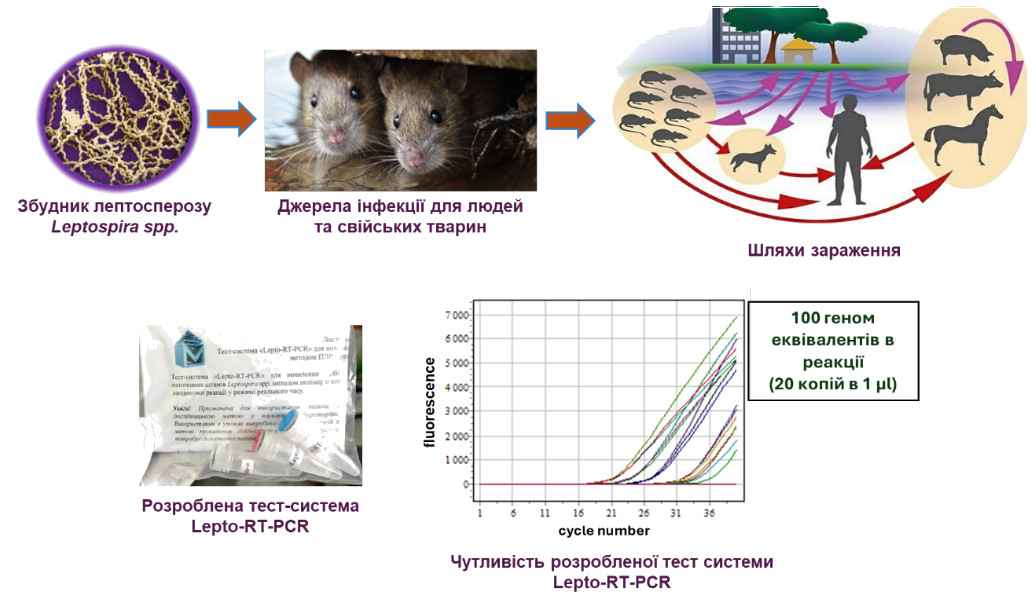
In close collaboration with other departments, a prototype of the Lepto-RT-PCR test system was developed for the detection of DNA of pathogenic Leptospira spp. strains by real-time polymerase chain reaction (using the intercalating dye Krystal Green). The ability of the Lepto- RT-PCR prototype to detect up to 100 genome equivalents in the reaction has been demonstrated.