Development and investigation of luminescent materials and dyes for biomedical, analytical, and optoelectronic applications.
Material Design: Probes and Labels for Biomedical Applications
Novel advanced red and near-infrared (NIR), extremely bright and photostable probes and labels for biomedical applications (Seta and Square series), fluorescence lifetime (FLT) markers (Seta and SeTau series), real dark quenchers (SQ series), and fluorescent classification (coding) dyes were developed and commercialized.
These materials are useful for biomedical and pharmaceutical assays based on fluorescence intensity, polarization, lifetime, or fluorescence resonance energy transfer (FRET). These materials, which have certain advantages over dyes of Cy, Alexa, DyLight, and ATTO series, include:
Nucleic Acids Intercalators and Novel Dimeric Dyes.
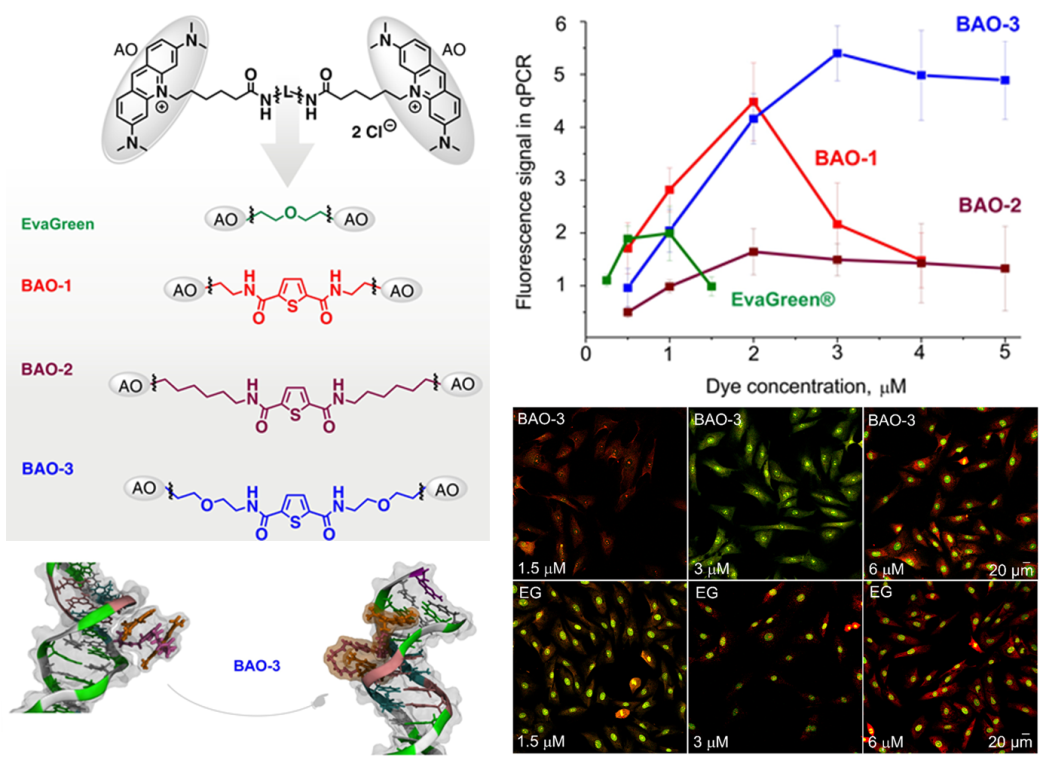
Recently, new nucleic acid-binding dyes based on bis-acridine orange (BAO) for nucleic acid detection and PCR analysis have been designed and synthesized. A comparative analysis of BAO dyes and the widely used DNA-binding dye EvaGreen® for fibroblast staining and qPCR analysis was performed. BAO dyes outperformed EvaGreen® by allowing qPCR amplification over a wider concentration range (0.5-5 μM) and generating consistent DNA melting curves, even at different DNA concentrations. Molecular dynamics simulations showed that upon binding to dsDNA, BAO dyes shift from a stacked to an elongated conformation, and this structural shift correlates with an increase in their fluorescence. These results deepen our understanding of the interaction of BAO dyes with dsDNA and confirm their potential for use in PCR and biovisualization.
Fluorescent Dyes as the Environment Sensors
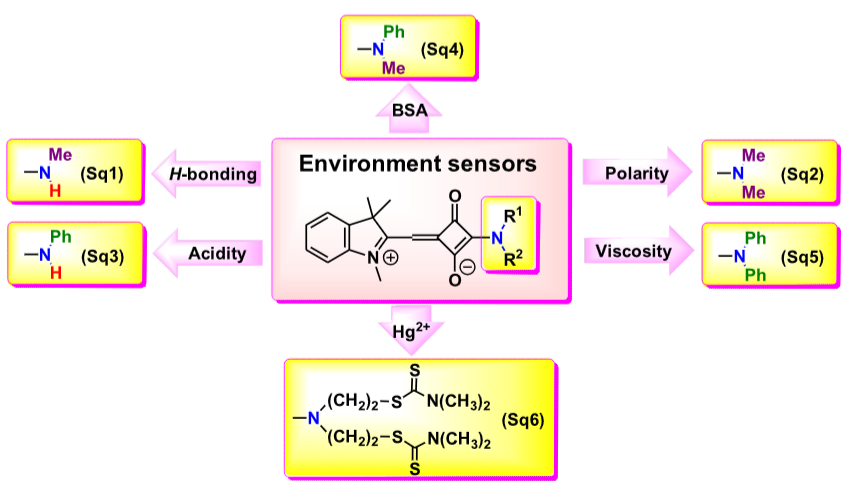
A series of push-pull hemisquaraine dyes (Sq1-Sq6) with different secondary and tertiary amino groups was synthesized. The type of amino substituent plays a key role in tuning the sensitivity of the dyes to hydrogen bonding, polarity, pH, viscosity, BSA, and Hg2+ ions, making them highly adaptive tools for sensing applications. The selectivity, sensitivity and other analytical parameters of such chemosensors are also studied in water, living cells and test systems.
Chemosensitive Nanoparticles Based on Polythienylboronic Acid
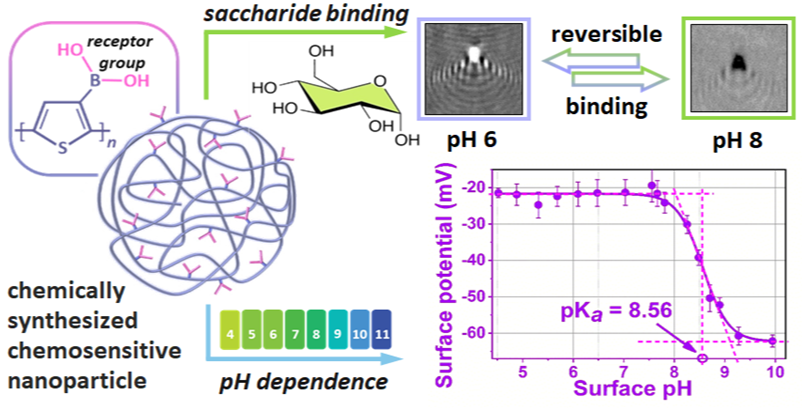
We have developed a scalable method for the synthesis of chemically sensitive poly-3-thienylboronic acid (PThBA) nanoparticles, which can be used for carbohydrate detection and controlled drug delivery. The nanoparticles have been thoroughly characterized and shown to be strongly dependent on pH and the presence of saccharide for their size, charge, and binding affinity. For the first time, a new method of plasmonic microscopy (WF-SPRM) was used to study the individual reactions of the nanoparticles in real time, which provided valuable information about their sensory properties.
Halogen-Containing Polymethine Dyes and Fluorophores for Anticancer and Antimicrobial Photodynamic Therapy
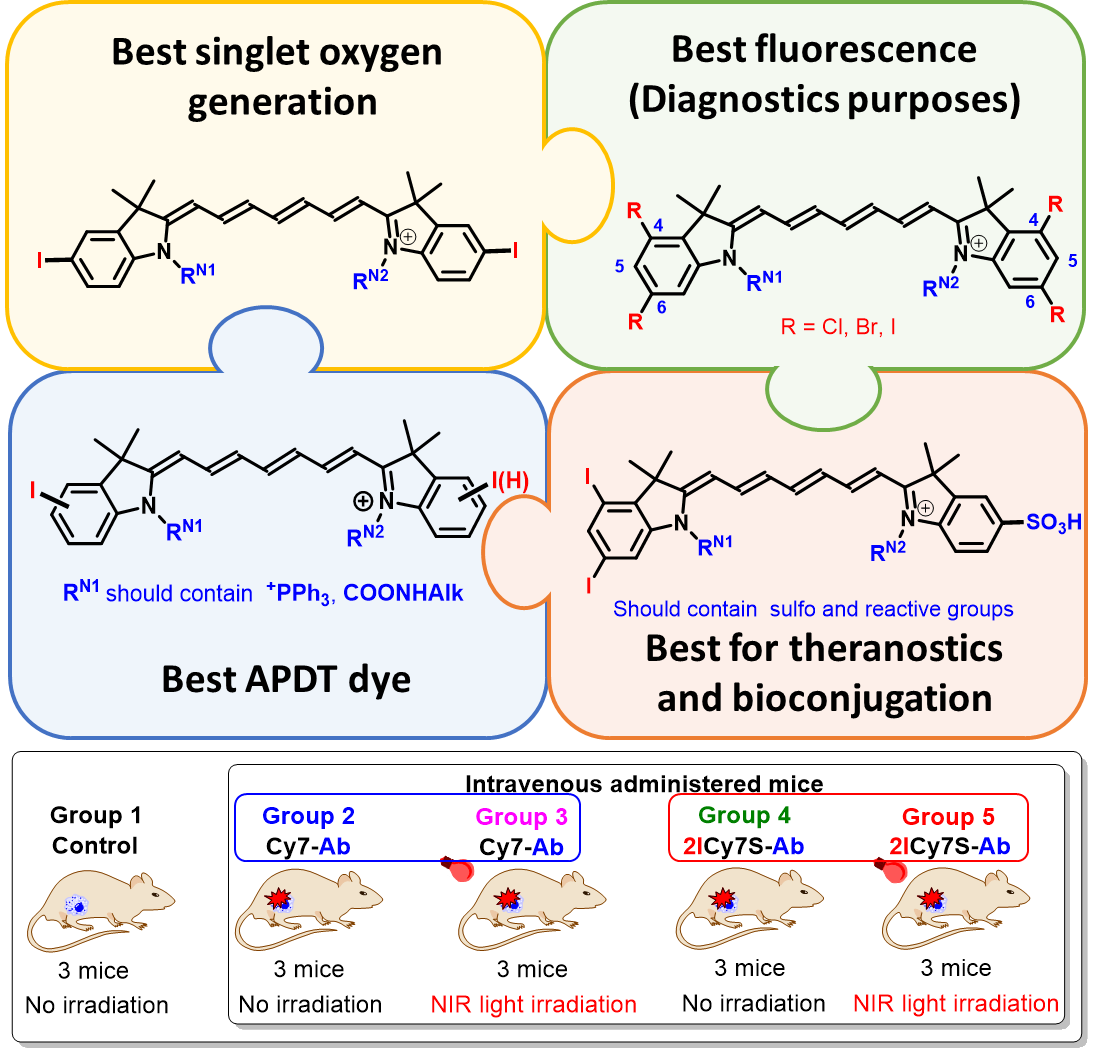
These studies are focused on solving key problems of development and application of halogen-containing polymethine dyes to create effective photosensitizers for anticancer and antimicrobial photodynamic therapy (PDT).
In the course of research on this topic, the following main tasks are being solved:
Bright and Stable Fluorescent Probes Based on Squaraine Rotaxanes and Other Supramolecular Architectures
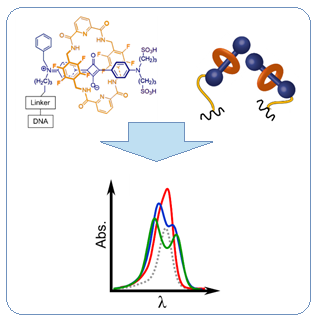
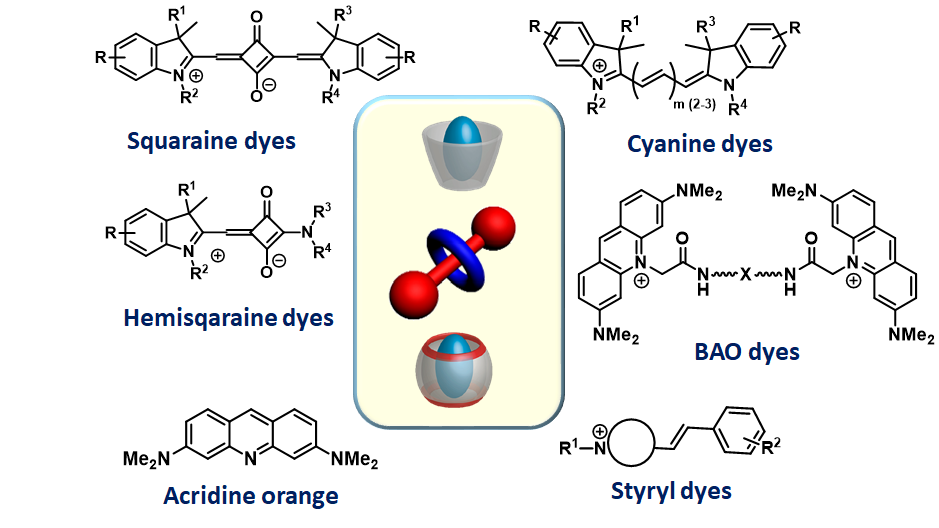
Bright, chemically and photochemically stable fluorescent dyes, probes and markers based on nanoencapsulated squaraines have been developed. The formation of supramolecular architectures, such as squaraine-rotaxane (SR) systems, significantly increases quantum yield, fluorescence lifetime and resistance to photobleaching and chemical degradation. These effects are due to the steric protection provided by the rotaxane structure, which protects the central squaraine fluorophore from external environmental influences. Notably, SR can adopt a rare oblique molecular packing that offers distinct advantages for applications in energy transfer, light harvesting, and quantum information processing. This packaging behavior is evidenced by the exciton-split absorption bands of almost equal intensity observed when two SR dyes are bound to DNA.
In addition to squaraine-based systems, supramolecular architectures based on hemisquaraine, cyanine, triphenylpyrazoline, and styryl dyes, as well as dimeric derivatives of acridine orange, are being created and studied. These materials are used for biomedical applications.